 Download this article in magazine layout
Download this article in magazine layout
- Share this article
- Subscribe to our newsletter
Countering the double-whammy of zoonotic diseases
A zoonotic disease (zoonoses) can infect both animals and people and be transmitted between vertebrate animals and people. Of the known (approximately 1,400) human pathogens 60 per cent have come from diseases that were first in animals. This historical trend has accelerated recently; of the newly emerging diseases in people approximately 75 per cent are believed to have come from animals. The virus that causes COVID-19, SARS-CoV-2, is the most recent example of a pathogen in animals then infecting people.
The magnitude of the problem
COVID-19 emergence has created the world’s first true pandemic for a hundred years. We are currently experiencing first-hand the health and economic burden of a pandemic born of a zoonotic ‘spill-over’ event. Zoonotic spill-over, the evolution of a pathogen from being wholly adapted to transmission between non-human animals to becoming wholly or partially adapted to humans, appears to be increasing in frequency. COVID-19, one of the most visible examples of zoonotic spill-over in recent history, follows the relatively recent emergence of Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS), Nipah virus, ‘Swine Flu’ and Highly Pathogenic Avian Influenza (H5N1), among others, as illustrated in the timeline below.
Timeline of significant emerging zoonoses outbreaks over the past 30 years
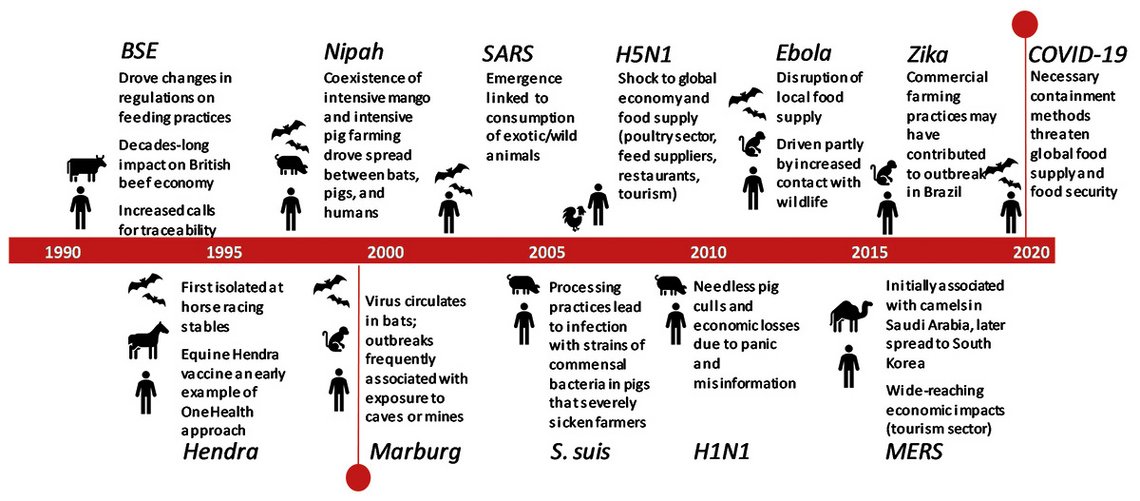
The significance of zoonoses with pandemic potential will not be lost on any readers; the huge cost in terms of human life and the economic shocks wrought by our response to this virus have firmly placed the risks of emerging diseases of zoonotic origin in the front and centre of public consciousness. The economic costs alone of emergence events are substantial, with six major zoonotic outbreaks occurring between 1997 and 2006 estimated to have had a combined economic burden of 80 billion US dollars. The final bill from the current COVID-19 pandemic will be in the trillions of dollars, alongside the significant health and mental suffering. In addition to the COVID-19 burdens, there are communities where other endemic zoonotic diseases that circulate constantly in people and their animals cause frequent and regular negative impact on economics, health, and wellbeing.
It is estimated that over two million people die yearly from endemic zoonoses. Millions more suffer from debilitating, chronic conditions that reduce their quality of life and their economic prospects, and often bring social isolation or stigma. The burden of zoonotic disease has been described as a ‘double-whammy’ where the human health impacts are exacerbated by losses suffered within the livestock sector, such as reduced productivity, livestock deaths, and the costs to farmers to control or treat these diseases. The burden of these zoonoses and of foodborne illnesses is felt predominately in low- and middle income countries (LMICs), within communities with least resilience to health and economic shocks. Endemic zoonoses are highly correlated with poverty by dint of their association with close contact between humans and livestock, poor sanitation, and inadequate access to preventative and curative health care. Consequently, these ‘neglected diseases’ of ‘neglected populations’ have historically been overlooked by policy-makers and healthcare providers alike.
What is driving the emergence of zoonotic diseases?
The chief causes of zoonotic spill-over and transmission within populations are many and varied; yet key drivers can be identified related to the increasing frequency of spill-over events, transmission of zoonoses and emergence of antimicrobial resistance (AMR) as illustrated in the Figure below. We can look at these drivers through the lens of our globalised food system and highlight aspects of current consumption, marketing and production of our food related to accelerating these events.
Interaction between intensification of livestock production, zoonoses & antimicrobial resistance
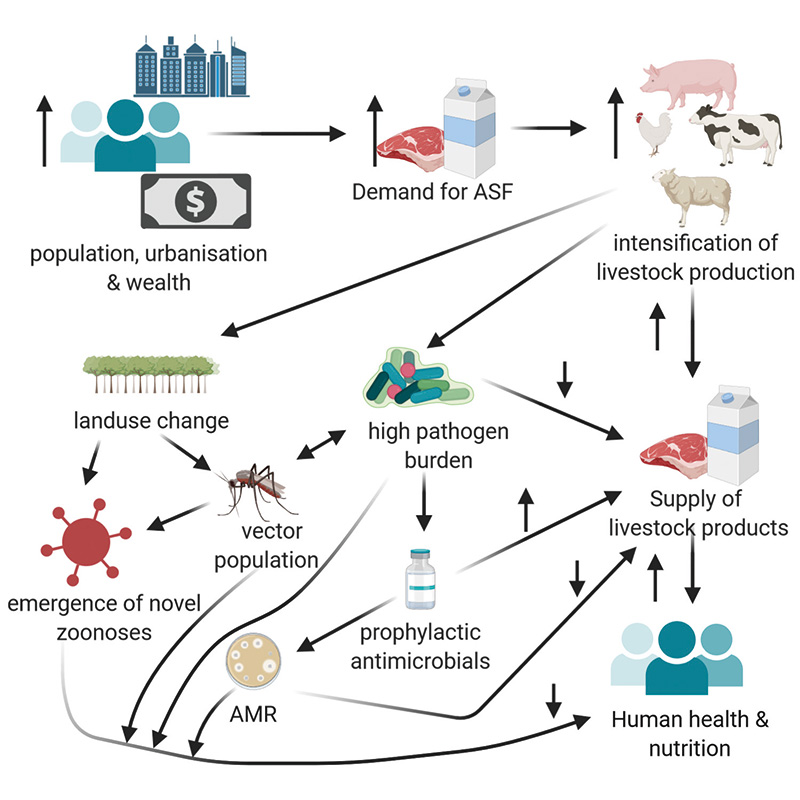
ASF = animal-source food Created with BioRender.com
Our growing and increasingly urbanised and affluent human population is driving an unprecedented expansion of agricultural production, specifically an increasing demand for animal-source foods. The location of the animal production units needed to meet this demand, or of the crop-lands required to provide feed input to these units, requires large-scale land use change, potentially encroaching into wildlife habitats and increasing the opportunities for contact between wildlife and humans or domestic livestock. It is estimated that between 2019 and 2050, up to one billion hectares of land will be newly converted into agricultural production. Alterations in vector distribution are also driven by other land-use changes such as widespread irrigation for rice crops, exacerbated by anthropogenic climate change (to which agriculture is a major contributor), resulting in increasing transmission of vector-borne diseases. Changing land-use and rainfall patterns appear to be responsible for altering temporal patterns of outbreaks of the mosquito-borne Rift-Valley Fever, whilst the mosquito vector of chikungunya and dengue, Aedes albopictus, has broadened its range northward, leading to the recent report of the first locally-acquired case of dengue in Italy.
Inadequate biosecurity practices along value chains provide opportunities for incursion of novel pathogens into the increasingly highly intensive system within them. Under intensified production systems, a high number of often genetically homogenous livestock species, or farmed-wildlife species, are kept in close proximity, potentially under conditions of physiological and psychological stress within which disease transmission between animals can be facilitated. Those working closely with these animals are at a high risk of acquiring infection with newly emerging diseases of animal origin.
The 1998 emergence of Nipah virus (NiV) in the Malaysian peninsula is an example of a virus arising from a wildlife reservoir (fruit bats) coming into contact with domestic livestock, through the co-existence of intensive mango and intensive pig production. This virus began circulating within the pig population and outbreaks of the virus, causing severe neurological disorders and with a 40 per cent fatality rate, occurred in workers in direct contact with infected pigs. Bat to human (via contaminated fruit) and human to human transmission has since been reported in Bangladesh and India. Similar co-existence of intensive poultry production with large populations of wildfowl harbouring Influenza H5N1 led to its emergence in China.
Intensification of livestock production has been historically heavily reliant on the use of antimicrobial agents for the prevention and control of disease often on a whole-herd/flock basis and, at sub-therapeutic levels, as antimicrobial growth promoters (AGPs). The sub-therapeutic use of antimicrobials results in selective pressure for resistant bacterial strains and agriculture-associated antimicrobial resistance (AMR) is of increasing concern world-wide, as we are seriously faced with the potential of a post-antibiotic future.
The onward processing, marketing and consumption of animal-source foods, both domestic livestock and wild-caught or farmed wildlife species, can also be responsible for potential zoonotic transmission events. It is hypothesised that the virus that causes COVID-19 was originally a pathogen of pangolins, the most extensively trafficked wild mammal in the world today, and the wet market, with its multitude of disparate mammalian species, provided the ideal environment for adaptive changes resulting in the sustained human-to-human transmission we are now experiencing on a global scale.
The speed of introduction of novel pathogens into multiple countries is obviously highly correlated with the globalisation of travel and trade, as people, foods, animals and objects can travel across the world in a day. Our globalised supply chains involve food products undergoing processing stages in multiple countries or even continents, and in each location being exposed to pathogens. The Figure demonstrates some of the drivers of zoonoses and antimicrobial resistance.
Little Glossary
An endemic zoonotic disease is one which circulates at a consistent level within the community, being transmitted between animals or through contact with animal-source products and humans. Examples include bovine tuberculosis, a bacterial disease of cattle related to the agent causing human TB which can infect humans and cause many similar symptoms, brucellosis, a bacterial disease causing malaise, joint and muscle pain and a relapsing fever, and neurocysticercosis, a brain infection caused by the intermediate, cyst, stage of the pork tapeworm, which is a leading cause of acquired epilepsy in regions where the parasite is present.
Foodborne diseases are those following the ingestion of food contaminated with bacteria, viruses, parasites or chemical toxins. World-wide, the majority of foodborne illnesses are diarrhoeal diseases caused by agents such as Salmonella spp., Campylobacter spp. and Norovirus.
Vector-borne diseases are those transmitted to humans by the bite of an arthropod vector such as ticks, lice, mosquitos and fleas. Some may be transmitted by the vector between humans, such as Dengue and Malaria, while others may be carried by the vector from animals to humans, such as Lyme disease and Rift Valley Fever Virus.
What can be done to predict, prevent and control zoonoses?
One Health, the concept that the health of humans, non-human animals and the environment is intrinsically linked, encourages us as a community to think and act in a multi-sectoral, multi-disciplinary way. The concept is multifaceted and, at its broadest, can be applied as a lens to many of the world’s health and environmental problems, but is highly applicable to the control and prevention of zoonotic disease. Fewer human health practitioners will now say ‘zoo-what?’ when zoonoses are mentioned, and there will be no turning away from the urgent need to improve our ability to detect and respond to zoonotic spill-over events, but a greater degree of One Health thinking and acting is necessary to have substantial impact on emerging and endemic zoonoses and the ever present threat posed by antimicrobial resistance alike.
Preventing zoonotic spill-over, reducing transmission events and mitigating the health and economic impacts of these threats require a paradigm shift in the way we organise and legislate our food systems (see also article "Uniting One Health and food systems for a more sustainable and inclusive world") and the structure of our animal and human health systems.
Improved multi-disciplinary training is required for professionals within human, animal and environment health to allow for ease of communication between sectors. One Health student networks and specific training in One Health have sprung up globally in recent years, a trend which must be sustained and indeed accelerated. While the diagnosis and treatment of zoonotic diseases will be improved both through increased awareness by frontline workers, it also requires accelerated development of appropriate diagnostic tests which should be affordable and easy to apply, particularly in resource-constrained settings.
There is growing evidence of the need to conduct control programmes for zoonoses in a One Health manner, targeting pathogens in both the human and non-human hosts and the environment or vector species. It is essential that healthcare services take an integrated approach, whereby these control programmes are cross-sectoral and instead of being primarily ‘vertical’ programmes, which focus on a single pathogen, they encompass a wider range of pathogens. This will improve the efficiency of programmes and lead to more sustainable outcomes. Identifying and capitalising on synergies, such as between the control of zoonotic and non-zoonotic helminth infections through water and sanitation (WASH) programmes and mass drug administration (MDA) programmes, is a first step in developing more co-ordinated and cost-effective control programmes.
Disease surveillance systems allowing for the integration of data from the human, animal and environment sectors are an important aspect of both early-warning for novel emergence events but also for the prioritisation and control of endemic zoonoses, foodborne disease and antimicrobial resistance. Ensuring interoperability between systems set up for use by individual sectors will allow for faster response to disease events, facilitate inter-sectoral understanding and co-operation and eventually improved data sharing at international level through full engagement with the global health security agenda and the international health regulations. Models for such integrated systems exist, such as the Danish programme for surveillance of antimicrobial consumption and resistance in bacteria from farm animals, food and humans (DANMAP). Extensive evaluation of such systems, including the legislative and budgetary changes necessary to implement them, is critical if they are to be replicated across many countries. Ensuring that environmental data is also integrated into these systems is the next crucial step in creating truly ‘One Health’ systems. Appropriately allocating surveillance resources into the prediction and prevention of zoonoses can be improved through the use of risk-mapping activities integrating socio-economic indicators, land use change, climatic data and host density and diversity.
Undertaking surveillance and control programmes and improving treatment of zoonoses is only one side of the coin. If we are to truly mitigate the burden of zoonoses in all their forms, we must simultaneously concentrate on addressing the underlying drivers. As a global community, we have to address sustainability of agricultural value chains and make health the central focus of our agri-food policies, including those relating to land-use planning, pharmaceutical use within livestock production, biosecurity, irrigation and waste management. These changes will also involve fundamental shifts in consumer perceptions and demands. Structural changes are needed to improve access to animal-source foods to those whose diets are fundamentally deficient in the valuable proteins and micronutrients they provide, whilst moving many of the world’s more developed economies back towards predominantly plant-based diets as recommended by the Lancet-EAT Commission.
Defining prioritities is key
All of the steps above will require strong political will to create the enabling environment for change, including the provision of adequate resources. Methodological prioritisation of zoonotic disease is necessary to identify greatest threats, formulate action plans and justify spending. To ensure accurate assess of impacts and risk, input is needed from sectors beyond animal and human health, including agents involved in environmental health, business, trade and government. The US Centre for Disease Control (CDC) leads OHZDP (One Health Zoonotic Disease Prioritization) workshops to help entities prioritise their top zoonotic diseases of greatest concern and develop One Health oriented plans to address identified diseases. This process brings together representatives from animal, human, and environmental health and stakeholders from multiple sectors. The process involves five steps: selection of stakeholders and zoonoses to be ranked, development of 5-8 key criteria, development of a single categorical question per criterion, ranking of criteria, and ranking of zoonoses based on answers to weighted criteria. This process uses qualitative, semi-quantitative and quantitative methods to achieve these ends.
This tool was developed to meet the needs of those working in areas where quantitative data on zoonoses are scarce and ties between human and animal health are underutilised. It also facilitates equal input from all invested stakeholders, accommodates diversity of location, scale and purpose, acknowledges data limitations, and is quick to increase action. Since its launch in 2014, 25 states, regions and countries have conducted an OHZDP alongside CDC facilitators. Sixteen of these assessments have been conducted in Africa, but none in Europe. By using the same methodology in different regions, CDC investigators have been able to identify common themes, which may help inform global research and capacity building needs.
Sophisticated metrics for cost-benefit analyses
OHZDP is a strong advocacy tool, but allocation of adequate resources to the prediction, prevention and control of zoonoses within a world of competing interests also requires robust economic data on the cost-effectiveness or cost-benefit of alternative courses of action. Economic evaluation of One Health Interventions within the surveillance, control and response to zoonoses is crucial to developing a robust ‘Business Case’ for One Health, including important discussions regarding cost-sharing between human and animal and public and private sectors. These evaluations require consistent metrics by which the burden of diseases can be measured in both the human and non-human populations. Whilst metrics are available to measure human health outcomes, such as the disability adjusted life year (DALY) developed for the global burden of diseases study, quantifying the impacts of disease within differing hosts requires more sophisticated metrics where impacts from both sectors can be measured in an equivalent way. Two solutions to this problem have been proposed to date. The Zoonoses-DALY (zDALY) transforms economic losses in livestock into an ‘animal life equivalent’ based upon the time taken to recoup that loss in the specific geographic context. An alternative approach is the transformation of human health burden into economic terms using the value of statistical life (VSL).
While substantial progress has been made to quantify the impacts of some zoonoses and foodborne illnesses, considerable gaps remain, particularly regarding the burden of disease in animal populations, and the impact of AMR on both humans and animals. Undertaking the robust, systematic collection, analysis and dissemination of this data is the founding mission of the Global Burden of Animal Diseases study (GBADs). This ambitious study will be undertaken by a large collaboration of academic partners with the support of the Bill and Melinda Gates Foundation, UK’s Foreign, Commonwealth & Development Office (FCDO), Australian Centre for International Agricultural Research (ACIAR), the equine welfare NGO Brooke, World Organization for Animal Health (OIE) and the UN Food & Agriculture Organization (FAO).
It is important, however, that further dimensions are integrated into our frameworks, including the social dimensions of human and animal disease and capturing environmental impacts. A full appreciation of the wider impacts of zoonoses is likely needed for the large-scale transformation of food, health and animal health systems which are required to move into a more sustainable, safe and food-secure world fit for habitation by both nine million people and the wide diversity of non-human life which our planet sustains.
Lian Thomas is a veterinarian and epidemiologist working at the Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, United Kingdom and the International Livestock Research Institute (ILRI). She is currently theme lead for Neglected Zoonoses in OHRECA and is a Soulsby Foundation One Health Fellow.
Grace Patterson is a microbiologist and epidemiologist studying food systems and health at the University of Liverpool’s Centre of Excellence for Sustainable Food Systems.
Lucy Coyne is a veterinarian and epidemiologist with research interests in antimicrobial resistance and policy. She is currently working in government policy and is an honorary postdoctoral research associate at the Institute for Infection, Veterinary and Ecological Sciences, University of Liverpool.
Jonathan Rushton is a biologist and economist working at the Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool. He leads a University Centre of Excellence for Sustainable Food Systems and the Global Burden of Animal Diseases programme.
Contact: lianthomas1@gmail.com
References and further reading:
Eurosurveillance: First autochthonous dengue outbreak in Italy, August 2020
https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.36.2001606?TRACK=RSS
DANMAP – Danish Programme for surveillance of antimicrobial consumption and resistance in bacteria from food, food and humans
https://www.danmap.org
EAT-Lancet Commission: Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems.
https://www.thelancet.com/commissions/EAT
Plos One: Prioritizing Zoonoses: A Proposed One Health Tool for Collaborative Decision-Making.
https://doi.org/10.1371/journal.pone.0109986
Patterson, Grace T. et al.: Moving health to the heart of agri-food policies; mitigating risk from our food systems
https://www.sciencedirect.com/science/article/pii/S221191242030078X?via%3Dihub
UNEP: Preventing the next pandemic – Zoonotic diseases and how to break the chain of transmission
https://www.unep.org/resources/report/preventing-future-zoonotic-disease-outbreaks-protecting-environment-animals-and
International Food Policy Research Institute: Mitigating health risks in sustainable agricultural intensification
https://www.ifpri.org/publication/mitigating-health-risks-sustainable-agricultural-intensification
Centers for Disease Control and Prevention: Emerging infectious diseases. Prioritizing Zoonoses for Global Health Capacity Building – Themes from One Health Zoonotic Disease Workshops in 7 countries, 2014-2016
https://wwwnc.cdc.gov/eid/article/23/13/17-0418_article





Add a comment
Be the First to Comment